
US advisers endorse single-shot COVID-19 vaccine from J&J
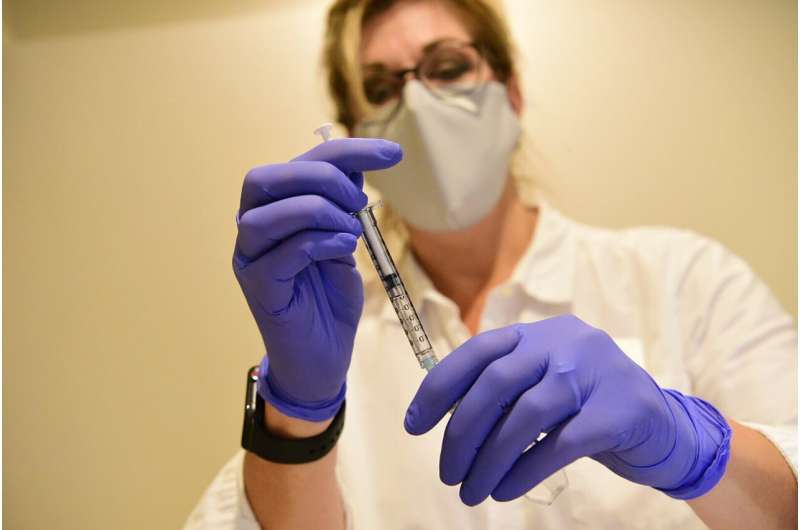
U.S. health advisers endorsed a one-dose COVID-19 vaccine from Johnson & Johnson on Friday, striking the nation on the cusp of adding a better-to-use option to fight the pandemic.
The performing head of the Food and Drug Administration talked about in an announcement that the agency will tear fast to discover the advice, which would possibly maybe perchance fabricate J&J’s shot the third vaccine authorized for emergency use within the U.S. Vaccinations are selecting up velocity, but modern gives are urgently wished to protect earlier than a mutating virus that has killed extra than 500,000 People.
After daylong discussions, the FDA panelists voted unanimously that the advantages of the vaccine outweighed the hazards for adults. Once FDA points a final resolution, shipments of a number of million doses would possibly maybe well originate as early as Monday.
“There’s an urgency to score this performed,” talked about Dr. Jay Portnoy of Kid’s Mercy Successfully being facility in Kansas Metropolis, Missouri. “We’re in a flee between the virus mutating—and modern variants popping out that can space off additional disease—and stopping it.”
Greater than 47 million other folks within the U.S., or 14% of the population, fetch received no longer lower than one shot of the 2-dose vaccines from Pfizer and Moderna, which FDA authorized in December. But the tempo of vaccinations has been strained by limited gives and delays due to wintry weather storms.
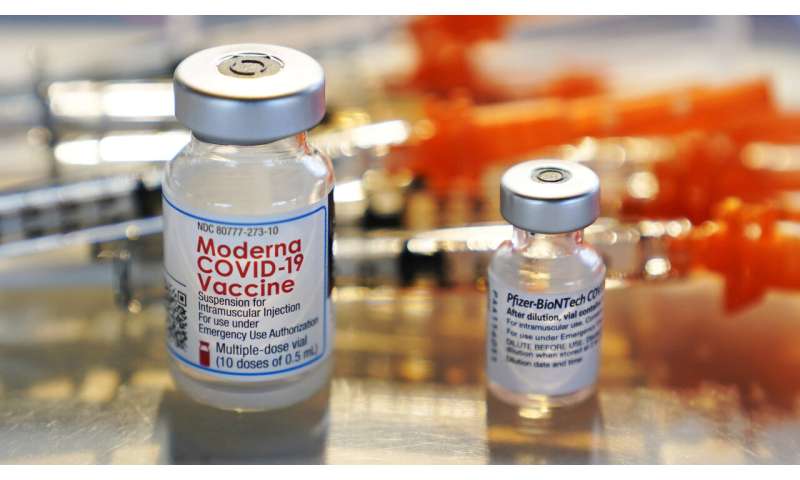
While early J&J gives will be limited, the corporate has talked about it’ll raise 20 million doses by the tip of March and a total of 100 million by the tip of June.
J&J’s vaccine protects against the worst outcomes of COVID-19 after one shot, and it would possibly maybe perchance even be stored as much as three months at fridge temperatures, making it more straightforward to accommodate than the previous vaccines, which fetch to be frozen.
One arena in rolling out the modern vaccine will be explaining how keeping the J&J shot is after the astonishing success of the first U.S. vaccines.
“Or no longer it’s important that folks comprise no longer think that one vaccine is better than every other,” talked about panelist Dr. Cody Meissner of Tufts College.
The two-dose Pfizer and Moderna shots had been chanced on to be about 95% effective against symptomatic COVID-19. The numbers from J&J’s study are no longer that excessive, but it be no longer an apples-to-apples comparison. One dose of the J&J vaccine modified into as soon as 85% keeping against the most extreme COVID-19. After adding in moderate instances, the total effectiveness dropped to about 66%.
Some consultants pain that lower number would possibly maybe well feed public perceptions that J&J’s shot is a “2d-tier vaccine.” But the variation in protection reflects when and the save J&J performed its reports.
J&J’s vaccine modified into as soon as examined within the U.S., Latin The US and South Africa at a time when extra contagious mutated versions of the virus had been spreading. That wasn’t the case final tumble, when Pfizer and Moderna had been wrapping up testing, and it be no longer certain if their numbers would retain against the most worrisome of those variants.
Importantly, the FDA reported this week that, ravishing bask in its predecessors, the J&J shot affords strong protection against the worst outcomes, hospitalization and death.
While J&J is looking out for FDA authorization for its single-dose model, the corporate is moreover studying whether a 2d dose boosts protection.
Panel member Dr. Paul Offit warned that launching a two-dose model of the vaccine down the avenue would possibly maybe well space off issues.
“You would watch the save that would possibly maybe well be confusing to other folks thinking, ‘Maybe I didn’t score what I wished,'” talked about Offit, a vaccine knowledgeable at Kid’s Successfully being facility of Philadelphia. “Or no longer it’s a ways a messaging arena.”
J&J representatives talked about they chose first of all the single shot since the World Successfully being Group and other consultants agreed it shall be a sooner, extra purposeful tool in an emergency.
Cases and hospitalizations fetch fallen dramatically since their January prime that adopted the wintry weather holidays. But public health officers warned that those gains will be stalling as extra variants steal root within the U.S.
“We are able to be performed with the virus, but clearly the virus is never any longer performed with us,” Centers for Disease Assign watch over and Prevention director Dr. Rochelle Walensky, talked about at some level of a White Home briefing Friday. She noted that modern COVID-19 instances fetch elevated over the last few days.
While it be too early to expose if the pattern will final, Walensky talked about adding a third vaccine “will motivate give protection to extra other folks sooner.” Extra vaccines are within the pipeline.

On Sunday, a CDC panel is predicted to meet to counsel how to best prioritize use of the J&J vaccine.
Other parts of the realm already are going whereby-is-best challenges. Italy’s important academics’ union recently protested when the authorities decided to direct Pfizer and Moderna shots for the elderly and designate AstraZeneca’s vaccine for youthful, at-possibility workers. AstraZeneca’s vaccine modified into as soon as deemed to be about 70% effective in testing. Canada turned the most modern country Friday to enable use of AstraZeneca’s vaccine.
© 2021 The Linked Press. All rights reserved. This arena fabric would possibly maybe well no longer be revealed, broadcast, rewritten or redistributed without permission.
Citation:
US advisers endorse single-shot COVID-19 vaccine from J&J (2021, February 27)
retrieved 28 February 2021
from https://medicalxpress.com/recordsdata/2021-02-endorse-single-shot-covid-vaccine-jj.html
This doc is arena to copyright. Rather than any ravishing dealing for the reason of non-public study or research, no
section will be reproduced without the written permission. The squawk material is geared up for recordsdata capabilities best.