ACIP: Health Workers, Lengthy-term Care Residents First for Vaccine
Editor’s point out: Obtain the most up-to-date COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
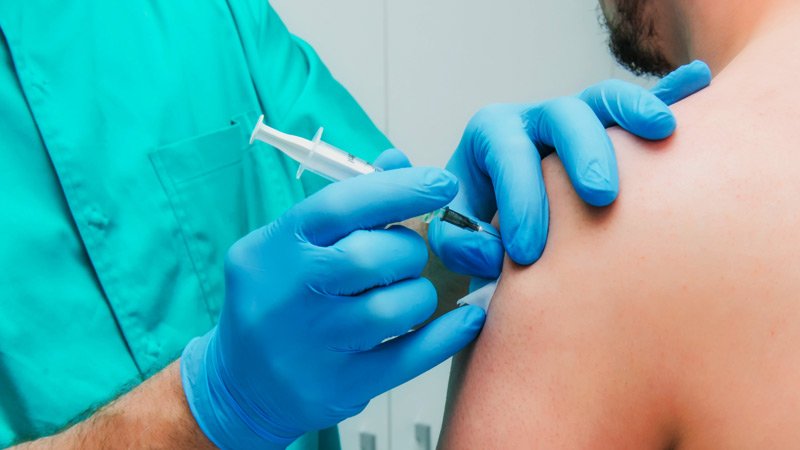
A federal advisory panel recommends that healthcare workers and residents of lengthy-term care facilities be the foremost to receive a COVID-19 vaccine when one is allowed for utilize by the US Food and Drug Administration (FDA).
The Amenities for Illness Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) voted 13-1 that each and each groups be within the very best likely-precedence community for vaccination. As such, ACIP recommends that each and each be included in portion 1a of the committee’s allocation thought.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. Express health departments are expected to rely upon the recommendation, but in a roundabout intention can create their beget decisions on how one can allocate vaccine of their states.
“We hope that this vote will get us all one step closer to the day after we can all feel protected all over again and when this pandemic is over,” stated Nancy Messonnier, MD, director of the CDC’s Nationwide Center for Immunization and Respiratory Diseases, at on the present time’s assembly.
Healthcare workers are outlined as paid and unpaid contributors serving in healthcare settings who’ve the likely of tell or oblique publicity to sufferers or infectious materials. Lengthy-term care residents are outlined as adults who dwell in facilities that provide a diversity of products and companies, including clinical and deepest care. Portion 1a would now not include kids who dwell in such facilities.
“Our aim in portion 1a near to health care personnel is to retain the group of workers and health care capacity no topic the build publicity happens,” stated ACIP panellist Grace Lee, MD, MPH, professor of paediatrics at Stanford College College of Treatment in California. Thus vaccination would veil scientific fortify workers, equivalent to nursing assistants, environmental products and companies workers, and food fortify workers, she stated.
“It is a actually considerable to preserve up our health care capacity,” stated ACIP member Sharon Frey, MD, scientific director on the Center for Vaccine Construction at Saint Louis College College of Treatment in Missouri. “Nonetheless it completely’s also well-known to forestall severe disease and death within the neighborhood that is at best likely possibility of those concerns and that entails those in lengthy-term care facilities,” she stated.
CDC workers stated that workers and residents in those facilities memoir for six% of COVID-19 cases and 40% of deaths.
Nonetheless Helen Keipp Talbot, MD, affiliate professor of remedy at Vanderbilt College in Nashville, Tennessee, voted in opposition to putting lengthy-term care residents into the 1a portion. “We have historically tried a vaccine in a young wholesome population and then hope it works in our extinct older adults,” she stated. “So we enter this realm of ‘We hope it works and that it be protected,’ and that concerns me on many levels in particular for this vaccine,” she stated, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who dwell in nursing properties or assisted living facilities.
She added, “I don’t have any reservations for health care workers taking this vaccine.”
Prioritization Could Alternate
The portion 1a allocation suits inner the “four ethical tips” outlined by ACIP and CDC workers November 23: to maximise advantages and decrease harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects most profit, minimal ruin, selling justice and mitigating the health inequalities that exist near to distribution of this vaccine,” stated ACIP Chair Jose Romero, MD. Romero, chief clinical officer of the Arkansas Division of Health, voted in prefer of the portion 1a thought.
He and other panelists considerable, nonetheless, that allocation priorities would possibly perchance well perhaps switch after the FDA opinions and authorizes a vaccine.
The FDA’s Vaccines and Associated Biological Products Advisory Committee (VRBPAC) will meet December 10 to examine the Pfizer/BioNTech’s messenger RNA-based entirely vaccine (BNT162b2). The companies filed for emergency utilize on November 20.
A 2d vaccine, made by Moderna, is now not a long way within the support of. The firm reported on November 30 that its messenger RNA vaccine became as soon as 94.1% effective and filed for emergency utilize the same day. The FDA’s VRBPAC will overview the protection and efficacy files for the Moderna vaccine on December 17.
“If particular individual vaccines receive emergency utilize authorization, we can have extra files to resolve into consideration, and that would possibly perchance well perchance lead to revision of our prioritization,” stated ACIP member Robert Atmar, MD, John S. Dunn Review Foundation Scientific Professor in Infectious Diseases at Baylor College of Treatment in Houston, Texas.
ACIP will meet all over again after the December 10 FDA advisory panel. Nonetheless it completely can also simply now not imply a product till after the FDA has authorized it, stated Amanda Cohn, MD, senior e book for vaccines on the CDC’s Nationwide Center for Immunization and Respiratory Diseases.
Staggered Immunization Subprioritization Informed
The CDC workers stated that given the likely that now not sufficient vaccine shall be on hand straight, it became as soon as recommending that healthcare organizations thought on rising a hierarchy of prioritization inner institutions. And, additionally they suggested staggering vaccination for personnel in similar items or positions, citing likely systemic or other reactions amongst healthcare workers.
“Possess in thoughts planning for personnel to have time a long way from scientific care if health care personnel expertise systemic symptoms post-vaccination,” stated Sarah Oliver, MD, MSPH, from the CDC.
The CDC will shortly be issuing guidance on how one can handle systemic symptoms with healthcare workers, Oliver considerable.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be on hand by the tip of December, with 5 to 10 million a week approaching-line after that, Cohn stated. Which intention now not all healthcare workers shall be vaccinated straight. That would require “sub-prioritization, but for a restricted time-frame,” she stated.
Messonnier stated that, even with restricted offers, various the states have suggested the CDC that they think they’ll vaccinate all of their healthcare workers inner 3 weeks — some in much less time.
The ACIP allocation thought is equivalent to but now not exactly much like that issued by the Nationwide Academy of Sciences, Engineering, and Treatment, which issued suggestions in October. That group stated that healthcare workers, first responders, older American citizens living in congregate settings, and people with underlying health stipulations must be the foremost to receive a vaccine.
ACIP has stated that portion 1b would include a actually considerable workers, including cops and firefighters, and people in education, transportation, and food and agriculture sectors. Portion 1c would include adults with high-possibility clinical stipulations and people used 65 years or older.
For additional news, apply Medscape on Facebook, Twitter, Instagram, and YouTube.