
It’s a have to-deserve to read this state while you’re jumpy about coronavirus vaccines
- Coronavirus vaccine study has advanced at swiftly stride, providing promising early results that indicate such medication would possibly maybe provide immunity and safety against severe illness.
- On the alternative hand, contemporary COVID-19 vaccine controversies will earn eroded the public’s believe in the modern medication.
- Researchers who had been following COVID-19 vaccine trend printed a paper that explains every thing that happened to this level this twelve months.
First and major of the radical coronavirus pandemic, successfully being consultants including Dr. Anthony Fauci acknowledged that a vaccine is maybe ready inner 12 to 18 months. That sounded gargantuan at the time, suggesting that scientists were somewhat assured of the odds of success. The months that adopted noticed extra than 100 coronavirus vaccines enter trials, with some of essentially the most promising ones having now advanced to Section 3 scientific trials.
The summer season brought us a giant surge in COVID-19 cases in the US, Brazil, India, Russia, and other nations throughout the enviornment, exhibiting the virus would no longer gradual down in warmer months cherish the flu. To find entry to to an efficient vaccine gave the affect indispensable extra mandatory, especially as some coronavirus study acknowledged immunity couldn’t final that long. The necessary Section 1 and Section 2 results came in for several of the advanced vaccine candidates, delivering promising news. The chances of success gave the affect high fascinated by that the series of experimental vaccine candidates jumped successfully over the 100 candidates that were registered in the principle few months of the pandemic. With all these varied solutions, completely some of them would possibly maybe work. And the extra functional vaccines we earn, the elevated the odds of assembly global quiz. The early results additionally showed that we would possibly maybe realistically query at the least one vaccine candidate to certain the final stage of human trials and be licensed by the FDA for emergency spend in gradual 2020.
However fair as vaccine study regarded as if it would exceed expectations, surveys started exhibiting that no longer all people changed into as soon as overjoyed at the prospect of getting a vaccine. A pair of months ago, one-third of Individuals who were surveyed acknowledged they’d no longer acquire a vaccine. That’s a foremost share, as resistance to vaccines would possibly maybe compromise heard immunity efforts. The most in fashion ballotshows that as many as two-thirds of People is rarely any longer going to acquire a COVID-19 vaccine, at the least no longer at the starting put. It’s no longer that the anti-vaccination circulation has obtained extra traction in the guts of a large pandemic, but contemporary controversies will earn had an instantaneous impact on the inhabitants’s enthusiasm for the drug. Even as you’re jumpy relating to the coronavirus vaccine trend though, here is the study paper it be a have to to investigate cross-check.
A pair of up-to-the-minute vaccine controversies would possibly maybe utter why some of us are jumpy about COVID-19 vaccination. All of it has to make with the stride of the study and trend of vaccines, seen thru the modern strikes that diverse nations earn made. Russia licensed its vaccine for emergency spend without exhibiting the enviornment any scientific data to indicate that it and not using a doubt works. China printed a total lot of analysis that shows several medication make induce the desired immune response without severe aspect-effects, however the nation has additionally licensed three vaccines for emergency spend sooner than Section 3 trials. Then reports came in announcing that the Trump administration would possibly maybe stress the FDA to flee its first coronavirus vaccine authorization so it coincides with the presidential election in November.
You don’t earn to be a die-laborious anti-vaxxer to gape all these developments and delivery being concerned relating to the safety and efficacy of vaccines. Going thru the available literature that particulars COVID-19 vaccine trend is maybe cumbersome and anxious, but that’s one choice to alleviate your concerns. You’ll acquire study on the total Section 3 medication, whether it’s Moderna, Pfizer/BioNTech, AstraZeneca/Oxford, or the three in the same trend advanced vaccine trials from China. Various study is supplied for vaccines which is maybe in preclinical or Section 1/2 trials. Novavax, Johnson & Johnson, and even Russia earn printed results for his or her medication.
However if there’s a single coronavirus vaccine-associated paper that that you just must read, it desires to be the Evolution of the COVID-19 vaccine trend panorama that changed into as soon as printed in Nature on Friday.
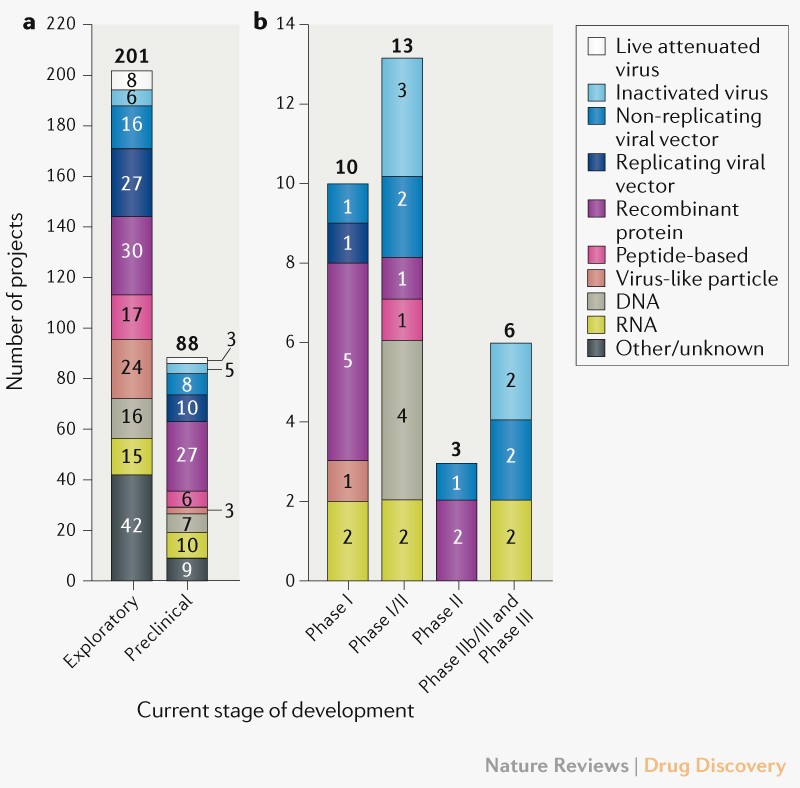
The paper offers the total data about COVID-19 vaccine work to this level, providing you with a mercurial hasten-thru of what’s occurring in the industry straight away. While the article can’t provide any form of guarantee that these vaccines will work, it restful offers a hopeful outlook that should counter the total alternative controversies where politics are fervent. The researchers additionally provide enter on what desires to be anticipated from COVID-19 vaccine trend, and what to scrutinize in upcoming vaccine data.
The researchers who compiled the facts utter that as of September 2nd, 321 vaccine candidates are in trend, when put next to 112 in April. Of these, 32 vaccine candidates are in scientific trials and so that they’ll enroll extra than 280,000 contributors from 470 sites in 34 varied nations. That’s to dispute the facts will seemingly be as tough as imaginable for medication that lengthen Section 3 and finis the study. The study that follow will provide certain data relating to the safety and efficacy of these medication.
The paper additionally explains the many vaccine applied sciences which had been stale to assemble COVID-19 medication. We’re searching at “a wide differ of craftsmanship platforms, including both primitive and new approaches.” The massive majority of these vaccines design the virus’s spike protein that binds to human cells, but other candidates lunge for varied targets. In other phrases, no longer simplest will we earn extra than 300 vaccines in trend, however the teams engaged on them are sorting out diverse systems of blockading the virus from infecting cells and/or lowering the severity of an infection. This would possibly maybe occasionally increase the odds that we’ll earn at the least some efficient vaccines in the near future, and that’s even supposing the modern Section 3 medication fail.
The researchers additionally label that “encouraging antibody and T cell responses had been reported for vaccines in preserving with several of the quite a total lot of platforms being stale,” but acknowledge that it’s too early to assess their relative ability. They additionally disclose that the presence of both of these formula of the immune design will seemingly be required to provide safety against COVID-19, so vaccines should induce both responses. Varied vaccines earn already proven that they’ll “manufacture” neutralizing antibodies and T cells.
In step with the paper, 11 medication reach from Chinese organizations and 7 are supported by Operation Warp Hurry in the US. The US authorities’s endeavor objectives to provide 300 million vaccine doses by January 2021, with extra than $10 billion already invested to strategy trend. Eight of these medication earn bought funding from the Coalition for Epidemic Preparedness Improvements (CEPI) and are integrated in the COVAX portfolio. That’s a collaboration between CEPI, the Gavi Alliance, and the World Health Group (WHO).
The paper additionally seems at the right scientific trend job, explaining the area of manufacturing a vaccine at some stage in an ongoing pandemic fueled by a new pathogen that desires further studying. Furthermore, the scientists utter what the endpoints, or goals, for the vaccine candidates desires to be. They utter that the design of vaccination trials should no longer be asymptomatic infections, as that form of end result couldn’t be measured adequately. An endpoint requiring indicators or symptoms of pneumonia would possibly maybe portray whether the drug works. That’s to dispute, if volunteers acquire infected but make no longer assemble pneumonia that can lead to severe respiratory issues, extra than one organ failure, and loss of life, then the vaccine is maybe deemed efficient.
Lastly, the paper notes that even supposing uncertainties stay about COVID-19 vaccine study, the principle Section 3 trial results should provide “in actual fact useful insights for the sphere and expose ongoing and future trend activities aimed no longer simplest at controlling the modern global pandemic but additionally for efficient long-duration of time immunization systems against the illness.”
The beefy paper is supplied in Nature.

Chris Smith started writing about objects as a keenness, and sooner than he knew it he changed into as soon as sharing his views on tech stuff with readers throughout the enviornment. Every time he is rarely any longer writing about objects he miserably fails to pause a ways flung from them, though he desperately tries. However that’s no longer essentially a unsuitable thing.