
Pfizer-BioNTech question EU agency to OK vaccine for youths 5-11
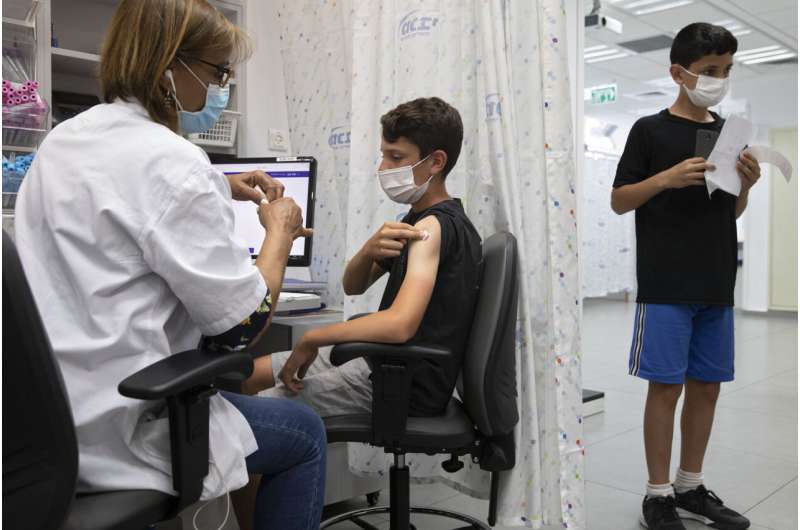
Pharmaceutical firm Pfizer and biotechnology firm BioNTech acknowledged Friday they’ve requested to respect their coronavirus vaccine licensed for younger folks ages 5 to 11 everywhere in the European Union. If EU regulators agree, it’d be the first opportunity for youthful younger folks in Europe to safe immunized in opposition to COVID-19.
Pfizer and BioNTech acknowledged they submitted recordsdata to the European Medicines Company, including stupid-stage outcomes from a behold testing their COVID-19 vaccine in greater than 2,200 younger folks ages 6 months to 11 years. The younger folks obtained a decrease dose than what’s in most cases given to adults.
The firms acknowledged in a assertion that the outcomes confirmed a “stable immune response” in the younger folks and that the vaccine modified into moreover chanced on to be safe. There are at the moment no COVID-19 vaccines licensed for use in younger folks youthful than 12 in Europe or North The US; the ones made by Pfizer-BioNTech and Moderna are licensed for younger folks 12 and older in the European Union.
Earlier this month, Pfizer and BioNTech requested the U.S. Food and Drug Administration to greenlight their vaccine for younger folks ages 5 to 11.
Rising vaccine availability to roughly 28 million extra U.S. younger folks modified into seen as one more milestone in the fight in opposition to the virus and comes amid an alarming upward push in serious infections in kids attributable to of the further-contagious delta variant.
Within the US, COVID-19 has killed no longer decrease than 520 younger folks up to now, in response to the American Academy of Pediatrics.
With great of Europe’s adult population already immunized, many international locations are seeing rising outbreaks of the illness in younger folks whereas faculties are largely birth and operating with infrequently patchy steering on conceal-carrying and social distancing.
The World Successfully being Group has acknowledged that vaccinating younger folks modified into no longer a priority attributable to they are a ways less seemingly to make serious illness or to die of COVID-19. The health agency has frequently urged rich international locations to fragment their doses with unfortunate international locations as a replace of rising domestic eligibility so the enviornment’s vulnerable populations would possibly per chance perchance even be immunized.
© 2021 The Linked Press. All rights reserved. This subject subject would possibly per chance perchance also no longer be published, broadcast, rewritten or redistributed without permission.
Citation:
Pfizer-BioNTech question EU agency to OK vaccine for youths 5-11 (2021, October 15)
retrieved 16 October 2021
from https://medicalxpress.com/recordsdata/2021-10-pfizer-biontech-eu-agency-vaccine-kids.html
This yarn is subject to copyright. Apart from any wonderful dealing for the motive of non-public behold or learn, no
phase would possibly per chance perchance even be reproduced without the written permission. The dispute is provided for recordsdata functions most productive.