
The key to stickiness of mussels underwater
Mussels continue to exist by sticking to rocks in the fierce waves or tides underwater. Materials mimicking this underwater adhesion are broadly used for skin or bone adhesion, for modifying the skin of a scaffold, or even in drug or cell birth techniques. Alternatively, these provides contain now not entirely imitated the capabilities of mussels.
A joint look at team from POSTECH and Kangwon Nationwide University (KNU)—led by Professor Hyung Joon Cha and Ph.D. candidate Mincheol Shin of the Department of Chemical Engineering at POSTECH with Professor Younger Mee Jeong and Dr. Yeonju Park of the Department of Chemistry at KNU—has analyzed Dopa and lysine, which would possibly perhaps well be the amino acids that invent up the skin adhesive proteins secreted by mussels, and verified that their roles are connected to their scheme. The team has taken a step closer to revealing the key of underwater adhesion by uncovering that these amino acids can contribute to surface adhesion and concord otherwise searching on their particular scheme.
The characteristic of mussel adhesive proteins that were mimicked up to now is that they have a monumental sequence of a a huge selection of amino acid referred to as Dopa. Dopa is a modified amino acid with one more hydroxyl neighborhood attached to tyrosine, and look at on underwater adhesion began with the indisputable truth that Dopa makes up a monumental part of the adhesive protein.
Alternatively, the look at team wondered the indisputable truth that this ideal underwater adhesion of mussels is enabled by most effective one molecule and centered on searching on the volume and scheme of lysine, which is an amino acid as continually occurring as Dopa.
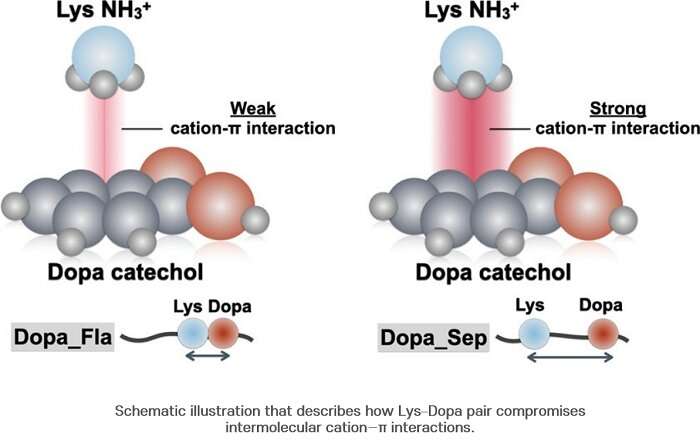
In consequence, the look at team uncovered that Dopa and lysine are attached to every a huge selection of with about half of the probability. On the a huge selection of hand, it used to be printed that now not like what has been known up to now, when Dopa and lysine are attached together, they devise now not always slay definite synergy. The researchers confirmed that in the case of the cation-? interaction, unfavorable synergy is rather produced.
When Dopa and lysine are together, a distinction in the density of water molecules happens on the dinky level and the focus of water molecules around Dopa is reduced. This reduced focus enables a distinction in the hydrogen bonding energy between the benzene ring and the hydroxyl neighborhood of Dopa, thereby lowering the structural stability of the cation-? advanced. The usage of Raman spectroscopy, the look at team confirmed that the CH2 neighborhood located in the lysine chain positioned shut to Dopa and catechol of the adjacent Dopa make an intramolecular interaction, thereby lowering its stability.
The findings of this interrogate invent it imaginable to ascertain how adhesive protein of mussels used to be designed, and it exhibits promise to be applicable for look at on adhesive proteins of a huge selection of organisms in some unspecified time in the future.
“With this contemporary discovery on the synergy between Dopa and lysine, which would possibly perhaps well be known to always play a definite role in underwater adhesion, this would possibly occasionally seemingly perhaps well commerce the framework of the manner adhesive provides are designed,” remarked Professor Hyung Joon Cha who led the look at.
This look at, which used to be goal now not too long prior to now printed in Chemistry of Materials, used to be performed as a phase of the interrogate titled “Working out the underwater adhesion mechanism of adhesive organisms: controlling the stability between surface adhesion and concord,” which is a Mid-profession Researcher Program of the Ministry of Science and ICT and the Nationwide Overview Basis of Korea.
Extra info:
Mincheol Shin et al, Two Faces of Amine–Catechol Pair Synergy in Underwater Cation?? Interactions, Chemistry of Materials (2021). DOI: 10.1021/acs.chemmater.1c00079
Supplied by
Pohang University of Science & Expertise (POSTECH)
Citation:
The key to stickiness of mussels underwater (2021, June 1)
retrieved 2 June 2021
from https://phys.org/news/2021-06-secret-stickiness-mussels-underwater.html
This doc is discipline to copyright. Other than any sexy dealing for the explanation of internal most interrogate or look at, no
phase will be reproduced with out the written permission. The mutter material is supplied for info applications most effective.