
X-rays dimension up protein construction at the ‘coronary heart’ of COVID-19 virus
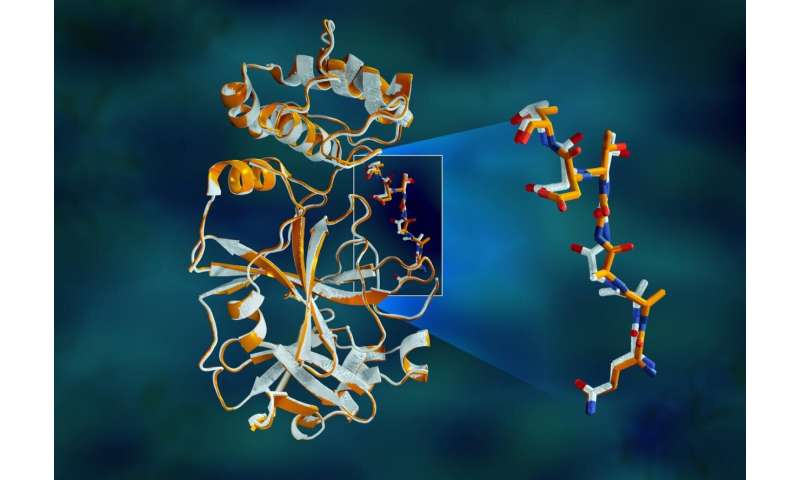
A crew of researchers at the Department of Energy’s Oak Ridge and Argonne national laboratories has performed the first room-temperature X-ray measurements on the SARS-CoV-2 predominant protease—the enzyme that enables the virus to reproduce.
The X-ray measurements keep a predominant first step in the researchers’ closing plot of establishing a total three-D mannequin of the enzymatic protein. The mannequin will doubtless be former to come supercomputing simulations geared in direction of finding drug inhibitors to dam the virus’s replication mechanism and assist pause the COVID-19 pandemic. Their compare results are publicly on hand and had been published in the journal Nature Communications.
SARS-CoV-2 is the virus that causes the illness COVID-19. The virus reproduces by expressing long chains of proteins that wishes to be minimize into smaller lengths by the protease enzyme.
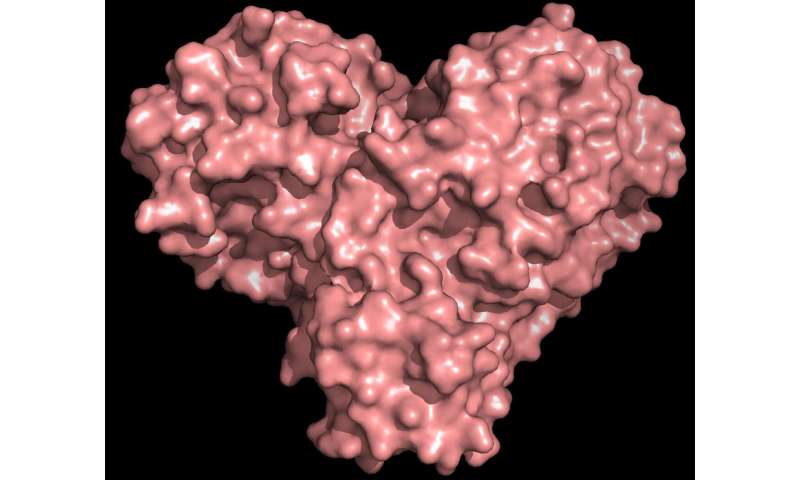
“The protease is well-known for the virus life-cycle. The protein is fashioned bask in a valentine’s coronary heart, but it undoubtedly undoubtedly is the coronary heart of the virus that lets in it to repeat and spread. Whenever you inhibit the protease and forestall the coronary heart, the virus can’t build the proteins which are important for its replication. That’s why the protease is regarded as the sort of predominant drug plot,” said ORNL’s Andrey Kovalevsky, corresponding author. While the come is known from cryogenically preserved crystals, “That is the first time the come of this enzyme has been measured at room temperature, which is predominant on tale of it is stop to the physiological temperature where the cells operate.”
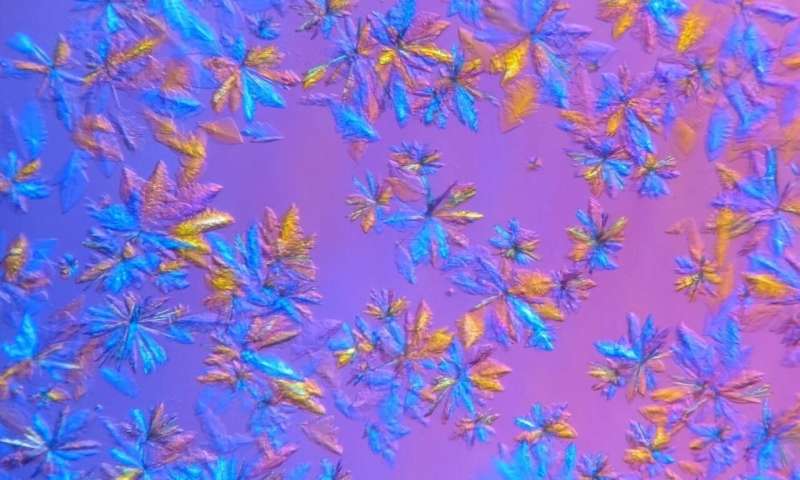
Constructing a full mannequin of the protein construction requires identifying every ingredient at some level of the come and how they’re arranged. X-rays are perfect for detecting heavy facets such as carbon, nitrogen and oxygen atoms. On tale of the intensity of the X-ray beams at most tall-scale synchrotron products and services, biological samples normally wishes to be cryogenically frozen to around 100 Ok, or approximately minus 280 levels Fahrenheit, to withstand the radiation long ample for knowledge to be silent.
To enhance the lifetime of the crystallized protein samples and measure them at room temperature, ORNL researchers grew crystals better than required for synchrotron cryo-experiences and former an in-dwelling X-ray machine that facets a less intense beam.
“Rising protein crystals and collecting knowledge is a late and time-drinking route of. In the time it normally takes to prepare and ship the sample to a synchrotron, we had been in an area to grow the crystals, resolve the measurements and commence analyzing the files,” said ORNL’s Daniel Kneller, the see’s first author. “And, when there is a lethal disease with many scientists mobilizing to see this subject, there is now not a day to spare.”
The protease enzyme includes chains of amino acids with a repeating sample of nitrogen-carbon-carbon atoms that make the backbone of the protein. Facet teams of the amino acid building blocks, or “residues,” lengthen from every of the central backbone carbon atoms. The enzyme is folded into a selected three-D form, creating special pockets where a drug molecule would connect.
The see revealed predominant structural disparities between the orientations of the backbone and some of the residues in the room-temperature and cryogenic samples. The compare suggests that freezing the crystals may perchance perhaps well moreover simply introduce structural artifacts that can lead to a less proper determining of the protease construction.
The crew’s results are being shared with researchers, led by ORNL-University of Tennessee Governor’s Chair Jeremy Smith, who are conducting drug docking simulations the utilization of Summit at ORNL—the nation’s quickest supercomputer.
“What researchers are doing on Summit is taking known drug compounds and searching to computationally bind them to the key protease for drug repurposing, apart from searching to search out new leads into various doable drug candidates,” said ORNL corresponding author Leighton Coates. “Our room temperature knowledge is being former to build a extra proper mannequin for those simulations and improve drug produce actions.”
The researchers’ next step in finishing the three-D mannequin of the SARS-CoV-2 predominant protease is to use neutron scattering at ORNL’s High Flux Isotope Reactor and the Spallation Neutron Supply. Neutrons are important to in finding the hydrogen atoms, which play a serious unbiased in many of the catalytic functions and drug produce efforts.
The protease plasmid DNA former to blueprint the enzyme turned into as soon as equipped by Argonne’s Structural Biology Heart at the Superior Photon Supply. Crystallization of the proteins former in the X-ray scattering experiments turned into as soon as performed at ORNL’s Heart for Structural and Molecular Biology.
Extra knowledge:
Daniel W. Kneller et al, Structural plasticity of SARS-CoV-2 3CL Mpro active keep cavity revealed by room temperature X-ray crystallography, Nature Communications (2020). DOI: 10.1038/s41467-020-16954-7
Quotation:
X-rays dimension up protein construction at the ‘coronary heart’ of COVID-19 virus (2020, June 25)
retrieved 26 June 2020
from https://phys.org/knowledge/2020-06-x-rays-dimension-protein-coronary heart-covid-.html
This fable is subject to copyright. Other than any comely dealing for the reason of non-public see or compare, no
phase may perchance perhaps well moreover simply be reproduced without the written permission. The speak is geared up for knowledge functions finest.