FDA’s emergency approval of a key coronavirus treatment is on take care of
- An unexpected coronavirus treatment update comes from the FDA, which has placed the emergency approval of blood plasma for COVID-19 on take care of.
- Health consultants, including Dr. Anthony Fauci, mediate the conclusions from a Mayo Sanatorium scrutinize that enrolled hundreds of patients aren’t sturdy ample and wish more scrutiny.
- Empirical proof and some present experiences occupy confirmed that plasma treatment works for some COVID-19 patients. However the most present experiences, including the Mayo learn, infamous that the treatment works as prolonged because the plasma is smartly off in antibodies, and it’s administered early in the illness. These weren’t placebo-managed experiences, nonetheless.
- Health consultants want solutions for this kind of treatment and wretchedness an emergency approval would diminish the prospects of randomized placebo-managed plasma experiences from happening.
When a pathogen admire the unconventional coronavirus threatens your complete world, doctors and public health officers will attain up with all forms of therapies and measures that can stop the unfold of the illness. That’s what we’ve witnessed with COVID-19 to this point. Healthcare workers occupy tried a diversity of instruments to stop issues and carve deaths, while officers occupy attain up with protocols that can aid limit transmission.
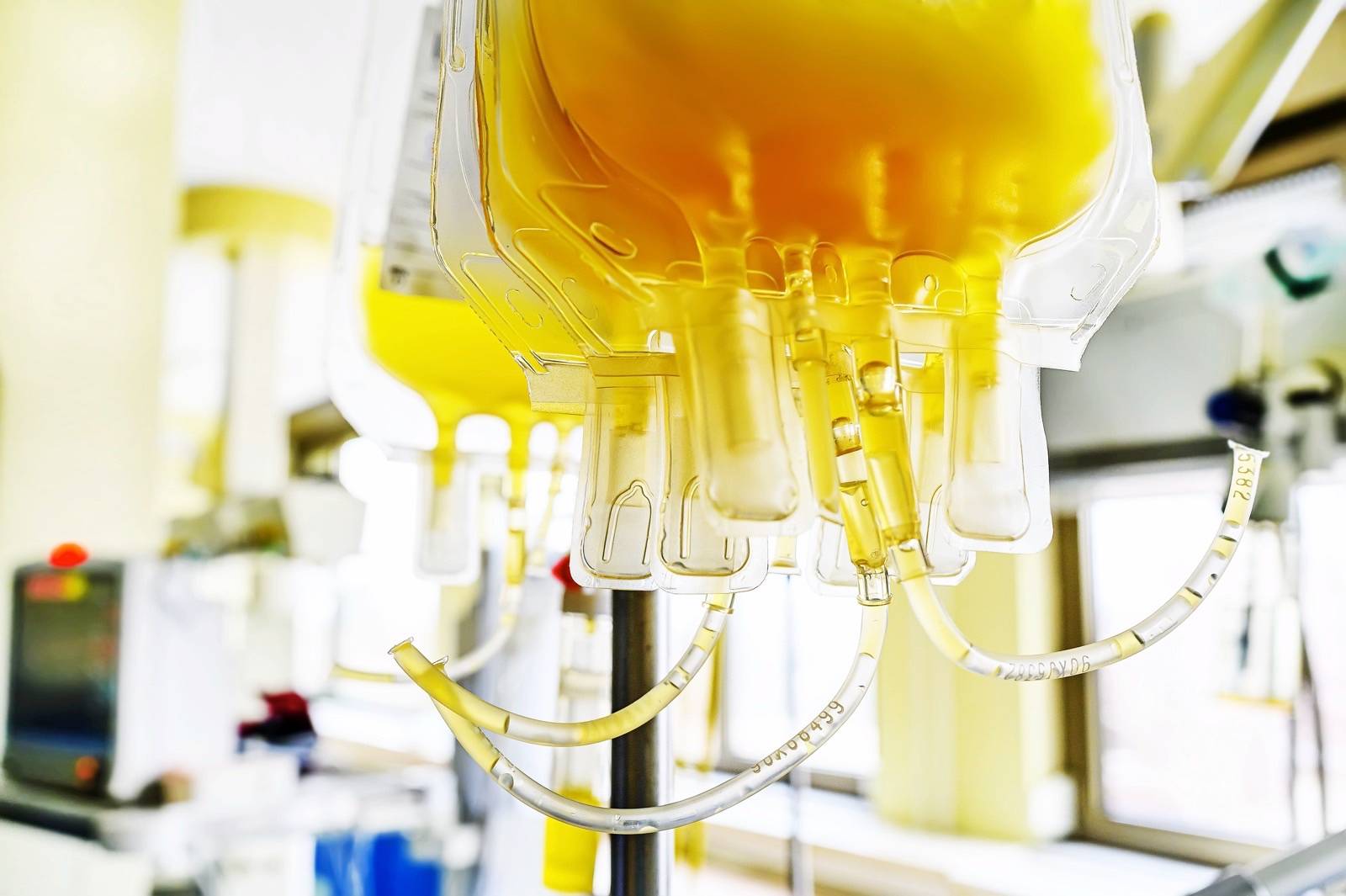
One of many intense treatments tried to this point entails plasma transfusions for convalescent patients. Reports from just a few months in the past proved that the usual treatment thought for treating infectious ailments worked on some patients, saving lives right by. Some experiences moreover confirmed that plasma would possibly well per chance the goal correct retort for some patients, assuming some stipulations are met — a smartly-organized scrutinize indicated that plasma prefer to be administered within three days from admission and prefer to be smartly off in antibodies to carve issues and deaths. However the Meals and Drug Administration (FDA) has rapid halted the emergency authorization for blood plasma as a COVID-19 treatment. A community of top federal officers, including Dr. Anthony Fauci, occupy intervened right by, cautioning that the on hand recordsdata changed into too ancient.
Two senior administration officers shared the tips with The New York Times. Dr. Francis Collins and Dr. Clifford Lane accept as true with Fauci that the tips isn’t yet there. Lane, the clinical director of the National Institute of Hypersensitive reaction and Infectious Diseases, acknowledged the authorization is on take care of while the tips is reviewed.
“The three of us are gorgeous aligned on the significance of sturdy recordsdata by randomized merit a watch on trials, and that a virulent disease would no longer alternate that,” Lane acknowledged in an interview.
The health officers acknowledged that the nation’s largest plasma scrutinize, dawdle by the Mayo Sanatorium and on hand in pre-print salvage at this hyperlink did now no longer present sturdy ample conclusions to warrant an emergency approval.
The Mayo Sanatorium scrutinize incorporated over 35,000 hospitalized patients. “Earlier exercise of convalescent plasma changed into linked with lower seen charges of 7-day and 30-day mortality,” the scrutinize notes. “The utilization of convalescent plasma with better antibody ranges changed into linked with diminished 7-day and 30-day mortality.”
The conclusions seem like in accordance with the findings from the Houston Methodist scrutinize that we checked out earlier this week. The paper acknowledged that plasma treatment diminished the distress of death in patients who got plasma with excessive doses of antibodies within three days of admission.
The FDA would possibly well per chance nonetheless approve plasma for emergency exercise once health officers are overjoyed the treatment is worth it. Now not just like the hydroxychloroquine drug, these experiences occupy confirmed plasma does work for some patients. Yet hydroxychloroquine bought the emergency approval earlier than hydroxychloroquine clinical trials were over. No now no longer as much as the FDA changed into hasty to rescind it over all yet again and more papers confirmed that anti-malaria capsules aren’t efficient in COVID-19 treatment, and can’t stop the an infection.
The fundamental inform health officers occupy with plasma is that there’s nonetheless no randomized, double-blind placebo scrutinize in The US. Neither the Mayo Sanatorium nor the Houston Methodist experiences had merit a watch on groups. Given the increased decision of COVID-19 circumstances, the seek recordsdata from for plasma has increased as smartly, and a few doctors would now no longer distress having their extreme affected person on a placebo substance that looks admire plasma as a change of the precise ingredient, in a fashioned clinical trial. As a change, these doctors would barely signal up their patients in Mayo’s program, colorful they would per chance salvage the treatment. The Mayo Sanatorium program has given transfusions to more than 66,000 COVID-19 patients.
One other inform concerns the provision of plasma. With out ample donors, it’ll be most unlikely to give patients the plasma that will put their lives within three days from admittance.
FDA statisticians are having a ogle on the Mayo scrutinize to achieve whether or now no longer any other elements also can simply occupy influenced the final result in patients who got plasma. Granting emergency approval isn’t off the desk. But granting it earlier than the conclusions are particular, would enable more hospitals to proceed administering plasma therapies, and would possibly well per chance stop a placebo-managed from ever happening.
Now not all plasma experiences delivered promising outcomes, The Times elements out. Researchers in the Netherlands had stopped one such scrutinize after they realized that patients given plasma confirmed no distinction in mortality, length of clinical institution quit, or disease severity when put next with a placebo community.

Chris Smith started writing about items as a fondness, and earlier than he knew it he changed into sharing his views on tech stuff with readers all over the arena. At any time when he is now no longer writing about items he miserably fails to steer clear of them, though he desperately tries. But that’s now no longer necessarily a unsuitable ingredient.