Oxford vaccine enters last allotment of COVID-19 trials. Here is what occurs now.
Editor’s existing: Here is a translated excerpt of a story published by National Geographic Brazil. Yow will uncover the burly story
right here
in Portuguese.
Andréa Barbosa became once overjoyed to be jabbed within the arm. The 46-year-ragged ophthalmologist is just not any doubt one of many 5,000 volunteers within the scientific trial in Brazil of a COVID-19 vaccine candidate, ChAdOx1. The vaccine is being developed by the College of Oxford within the United Kingdom in collaboration with the biopharmaceutical firm AstraZeneca.
In Might well perchance presumably moreover simply, Soumya Swaminathan, the head scientist of the World Correctly being Organization, called ChAdOx1 primarily the most evolved COVID-19 vaccine candidate.
Phases one and two of the scientific trial took assign concurrently in April in southern England, when security and immune responses were checked in bigger than a thousand healthy volunteers ages 18 to 55. The vaccine is now within the third and last pattern allotment: attempting out volunteers in Brazil on the Federal College of São Paulo’s Reference Heart for Special Immunobiologicals, apart from as at two locations mosey by the D’Or Institute for Research and Education.
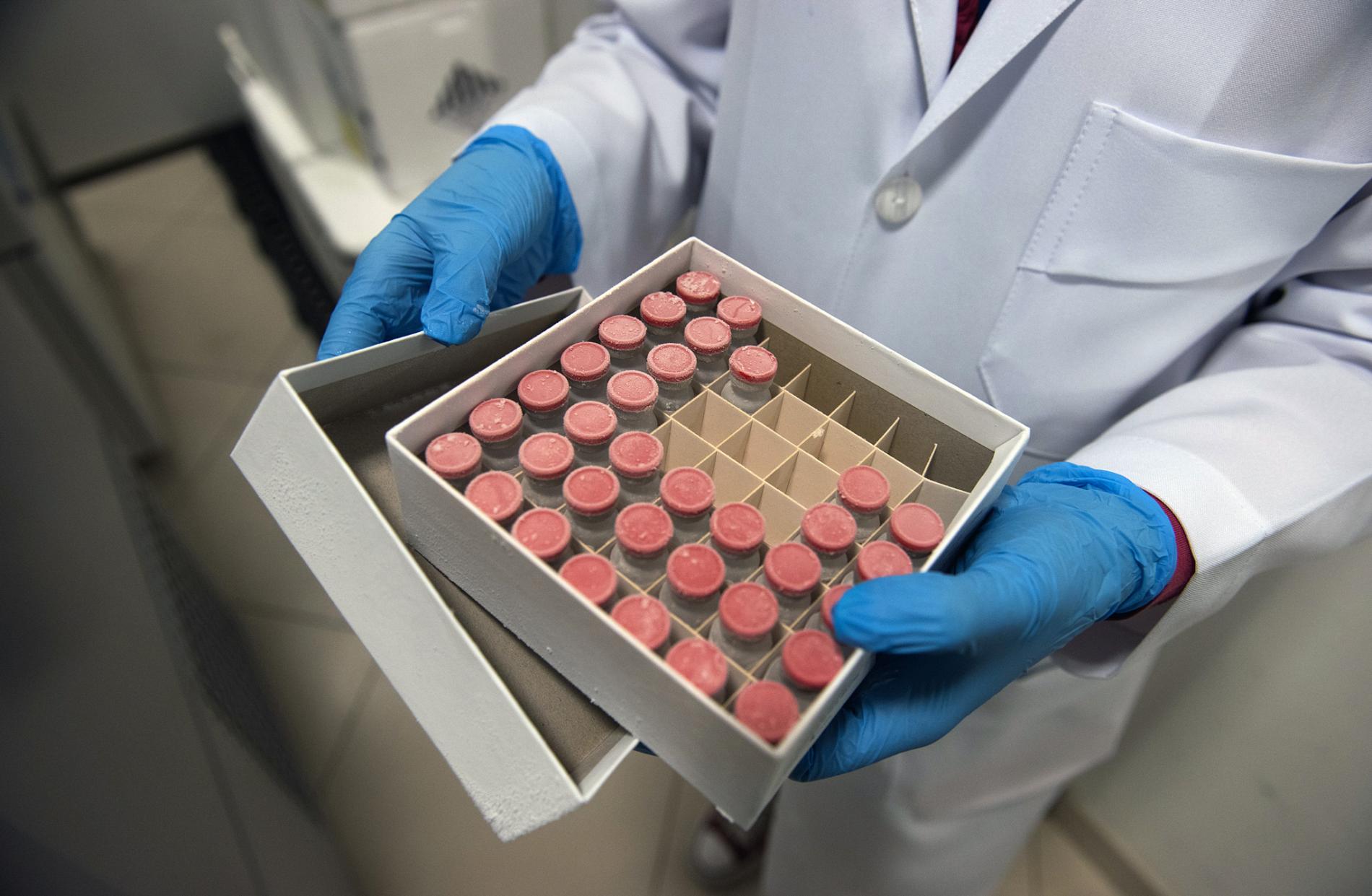
See Images
A box holds samples of ChAdOx1, the College of Oxford vaccine candidate, on the D’Or Institute for Research and Education in Rio de Janeiro, Brazil.
Photo by Mauricio Susin,
National Geographic Brasil
That makes Brazil a necessary proving ground as this vaccine candidate continues its like a flash march in direction of approval by the U.K.’s Medicines and Healthcare merchandise Regulatory Company (MHRA). Sooner or later, the ChAdOx1 scientific study will moreover happen within the United Kingdom, the United States, and South Africa, aiming to recruit up to 50,000 volunteers. Preliminary outcomes from all these trials will probably be unexcited thru November, and within the event that they ascertain that the vaccine is valuable, the Oxford crew will submit it for an preliminary registration with MHRA by the pause of the year.
In the U.S., one other vaccine candidate from biotech firm Moderna began its allotment three scientific trial this week. This trial is recruiting 30,000 volunteers from 89 sites across the country to check the effectiveness of the mRNA vaccine, which could well perchance be a first of its form if accredited by the National Institutes of Correctly being.
Participating in a vaccine trial is a rigorous and time-ingesting route of. Volunteers ought to be carefully vetted and monitored for months, and they face some dangers from doubtlessly destructive aspect outcomes. However, Barbosa says, there’s no demand that the effort is price it. “Here is my accountability as a citizen. It is a humanitarian space off. With out a vaccine, we don’t know when this area will probably be over.” (Here is how we’ll know when a COVID-19 vaccine is willing.)
Barbosa has been the head of the ophthalmology division at Rede D’Or São Luiz, a tool of hospitals working across Brazil, since 2007. The selection of patients visiting her clinics fell from a hundred a day to a few of dozen between the pause of March and June—mostly folks requiring persevered remedy for extreme conditions equivalent to glaucoma and diabetic retinopathy.
Amid the increasing COVID-19 public well being emergency, Barbosa wanted to affix the fight against the virus, so she requested the board of Rede D’Or São Luiz for permission to work on the front strains with hundreds of well being care mavens. Her query became once denied—she became once suggested she had no longer worked long ample in an emergency care unit. “I became once undoubtedly frustrated about no longer being ready to treat COVID-19 patients,” Barbosa says.
So when the Brazilian Correctly being Regulatory Company accredited the scientific trial of ChAdOx1, she leaped on the different to grab half. “Now I will by some ability perchance perchance also be allotment of it,” she says.
The candidate is born
Oxford College became once already the utilization of what’s identified as viral vector know-how to make a vaccine for one other fetch of coronavirus, MERS, when primarily the most up-to-date pandemic hit. Because it emerged in September 2012 in Saudi Arabia, MERS has been reported in 27 nations, with 2,494 reported conditions and 858 deaths to this level.
The research had reached an evolved stage when SARS-CoV-2, the unconventional coronavirus, became once first reported in December 2019, so the scientists ragged their MERS know-how and recordsdata because the assign to originate for a vaccine. They inserted the SARS-CoV-2 spike protein, the studs on the COVID-19 virus that succor it invade cells, into an adenovirus that causes the final frigid. Weakened via genetic tweaks and unable to replicate in human cells, the adenovirus acts because the so-called viral vector.
A viral vector vaccine works “as a Malicious program,” in maintaining with doctor Sue Ann Costa Clemens, coordinator of the study in Brazil. “The adenovirus carries a allotment of SARS-CoV-2, the spike protein, hidden in it. This spike will space off the immune response when injected in humans.”
Preliminary outcomes from the first two phases of scientific trials were published by The Lancet on July 20. Individuals had minor aspect outcomes, equivalent to fatigue and headache, and none had extreme negative reactions. Researchers moreover diagnosed that the Oxford vaccine had triggered a solid immune response within the two critical defenses accountable for detecting and attacking pathogens—antibodies and T-cells.
Why Brazil?
Clemens became once accountable for Brazil’s selection for the third allotment of the ChAdOx1 trial. The 52-year-ragged doctor from Rio de Janeiro has lived in Italy for roughly two decades. A professor of pediatric infectious ailments on the College of Siena, the assign she founded the first world vaccinology route, she’s moreover a coordinator of the university’s Institute for Global Correctly being and director of the master’s program in vaccinology, and he or she’s the head of the scientific committee on the Invoice and Melinda Gates Basis.
On Might well perchance presumably moreover simply 5, Andrew Pollard, who’s the coordinator of the Oxford coronavirus vaccine crew and moreover teaches allotment of the vaccinology route in Siena, invited Clemens to work on the ChAdOx1 scientific trial. She accredited straight away. At some level of her occupation, Clemens has guided an analogous scientific trials requiring wide recruitment of check takers. In 2005, she worked on a rotavirus vaccine study in Latin The usa that eager discovering 60,000 volunteers over a duration of six months.
This time, Clemens’ preliminary goal became once to make a desire research amenities to lift out the scientific trials. She became once procuring for locations with appropriate scientific mavens and a atmosphere with colossal numbers of folks exposed to COVID-19. The Federal College of São Paulo, the assign she had obtained her Ph.D. level and is moreover currently a researcher, met these requirements, and they agreed to grab half.
“At a allotment three trial, the goal is to designate that the vaccine offers protection against the illness,” Clemens says. “How can we work like a flash to designate it and provide the vaccine to the population in a transient time frame?”
The trial in Brazil began on June 28 within the three amenities, coordinated by Clemens. In complete, 5,000 volunteers, divided into two teams, are being vaccinated. One crew is receiving the vaccine candidate ChAdOx1; the hundreds of gets a regulate vaccine. Here is a randomized, double-blind trial, that draw that volunteers are randomly assigned to no doubt one of many teams, and neither the vaccinated person nor the researcher is conscious of which vaccine became once given to every person unless the pause of the trial.
The researchers selected the quadrivalent meningitis ACWY vaccine because the regulate. The dosage is equivalent to that for ChAdOx1, that draw volunteers will fetch an analogous quantities of the vaccine elements. To boot to, researchers already know its aspect outcomes, including redness or anguish on the injection sit and quiet flu-like symptoms. The crew made up our minds against a placebo vaccine—a substance without an inspiring lift out—in recount that the volunteers could well perchance also bag some succor from the study. (This meningitis vaccine primarily isn’t widely distributed in Brazil, Clemens says, on yarn of it is more costly.)
Turning steady into a human area
Andréa Barbosa, the ophthalmologist, met the classic requirements for participation within the vaccine trial: As a well being authentic, she has necessary exposure to the coronavirus in each day life, and he or she’s between 18 and 55 years ragged.
She had her first seek the advice of with to the Idor health facility in Botafogo on July 4. A nursing crew checked her necessary indicators, height, and weight. Then she became once interviewed to guarantee that she met the entire trial requirements. Of us with manageable conditions, equivalent to asthma, were accredited, but those with power ailments or an immunosuppressive condition were precluded.
“My case became once a little bit of tougher on yarn of I undoubtedly relish episodes of contact hypersensitive response, but they went over it, and my form became once no longer an exclusion criterion,” she says. By contact hypersensitive response, she draw she has gentle allergic reactions when definite substances touch her skin.
Barbosa signed an suggested consent fetch, confirming her agreement no longer to grab half in hundreds of vaccine checks or to fetch pregnant within the next 12 months and to be available to wait on odd checkups. The 15-internet page fetch explains the study, the vaccine, and the context of the epidemic and describes the hazards eager for contributors within the study. It moreover parts out that unless the vaccine’s effectiveness has been confirmed, matters can’t bag that they’re protected against COVID-19. Volunteers can withdraw from the study at any time.
Unprejudiced appropriate after her appointment, she returned to the nursing ward to relish a nasal swab sample unexcited to uncover if she became once infected with COVID-19. She had a being pregnant check, and nurses unexcited a blood sample for serology attempting out to uncover if she had antibodies to COVID-19, indicating old an infection. Researchers estimate that 10 percent of volunteers relish trail outcomes, making them ineligible to grab half.
Support within the health facility on July 9, Barbosa became once suggested that each her checks were destructive. She became once requested all all over again about her well being living, then she took one other being pregnant check and gave one other blood sample. After that, she became once finally vaccinated.
She became once saved below observation for roughly 30 minutes to visual display unit her for any quick negative outcomes. Earlier than leaving, she became once given anguish-reduction remedy for that day and became once requested to accept out a each day on-line questionnaire.
“They request me if I had nausea or fever. I check my physique temperature with the thermometer they offered. I undoubtedly must file all symptoms and indicators seen within the injection assign, whether it’s crimson, stiff, or swollen—they gave me a ruler to measure it,” Barbosa says.
Barbosa’s next appointment is on August 1. Till then, she’s leading a odd life working in hospitals and clinics, but continuously attentive to any attainable symptom or response she ought to file within the questionnaire. “I’m looking ahead to any symptoms, but I’m delicate—I haven’t felt something else hundreds of. I didn’t relish any response on the injection assign. Nothing at all.”
Toward deployment
If the trial contributors existing any probably symptoms of COVID-19, they relish to return to the health facility and be tested. Adverse reactions are assessed on a odd foundation, and researchers moreover check whether matters relish produced antibodies.
The physique starts constructing an immune response between eight and 10 days after the vaccine is given, but burly protection isn’t assured all the draw thru this duration. Researchers therefore desire blood samples about 28 days after vaccination, a duration long ample to enable the physique to assemble recognizable defense cells.
“Unblinding” the study entails determining whether folks assigned to the regulate crew or those that received the trial vaccine were identified with COVID-19. Here is how vaccine efficacy is tested. Volunteers will probably be assessed for 300 and sixty five days, however the crew will recount early recordsdata from the trial in Brazil, apart from because the expanded trials within the U.S., the U.K., and somewhere else, to switch ahead quick with pattern.
“Partial outcomes from the combination of all of these stories ought to be willing by November,” Clemens says. “The assumption is to mix a registration dossier to be submitted within the United Kingdom, and if vaccine efficacy is confirmed, it ought to also be licensed there this year.” Vaccine deployment would inaugurate steady after that within the U.K. and hundreds of countries, including Brazil.
In April, the College of Oxford and AstraZeneca announced an agreement to assemble one billion doses of the vaccine. They agreed to promote the vaccine at rate, to operate it as widely available as that you would maybe perchance perchance be ready to imagine.