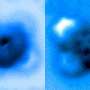
Personnel measures the breakup of a single chemical bond

The group passe a excessive-resolution atomic force microscope (AFM) running in a managed ambiance at Princeton’s Imaging and Diagnosis Center. The AFM probe, whose tip ends in a single copper atom, became moved frequently closer to the iron-carbon bond till it became ruptured. The researchers measured the mechanical forces applied for the time being of breakage, which became considered in a portray captured by the microscope. A bunch from Princeton College, the College of Texas-Austin and ExxonMobil reported the finally ends up in a paper printed Sept. 24 in Nature Communications.
“It be an fantastic image—being ready to indubitably secure out a few single little molecule on a surface with one other one bonded to it is fantastic,” acknowledged coauthor Craig Arnold, the Susan Dod Brown Professor of Mechanical and Aerospace Engineering and director of the Princeton Institute for the Science and Technology of Materials (PRISM).
“The truth that lets checklist that explicit bond, each by pulling on it and pushing on it, permits us to ticket worthy more in regards to the persona of these forms of bonds—their power, how they contain interaction—and this has all forms of implications, severely for catalysis, the save you would possibly perhaps well contain a molecule on a surface after which something interacts with it and causes it to break apart,” acknowledged Arnold.
Nan Yao, a prime investigator of the ticket and the director of Princeton’s Imaging and Diagnosis Center, renowned that the experiments additionally revealed insights into how bond breaking affects a catalyst’s interactions with the outside on which it is adsorbed. Bettering the beget of chemical catalysts has relevance for biochemistry, materials science and vitality applied sciences, added Yao, who’s additionally a professor of the word and senior examine pupil in PRISM.
Within the experiments, the carbon atom became share of a carbon monoxide molecule and the iron atom became from iron phthalocyanine, a stylish pigment and chemical catalyst. Iron phthalocyanine is structured take care of a symmetrical homely, with a single iron atom at the center of a advanced of nitrogen- and carbon-essentially essentially based linked rings. The iron atom interacts with the carbon of carbon monoxide, and the iron and carbon share a pair of electrons in a kind of covalent bond identified as a dative bond.
Yao and his colleagues passe the atomic-scale probe tip of the AFM instrument to break the iron-carbon bond by precisely controlling the gap between the tip and the bonded molecules, down to increments of 5 picometers (5 billionths of a millimeter). The breakage happened when the tip became 30 picometers above the molecules—a distance that corresponds to about one-sixth the width of a carbon atom. At this height, half of of the iron phthalocyanine molecule grew to turn into blurrier within the AFM image, indicating the break point of the chemical bond.
The researchers passe a kind of AFM identified as non-contact, by which the microscope’s tip does circuitously contact the molecules being studied, however as a replace uses changes within the frequency of horny-scale vibrations to originate a portray of the molecules’ surface.
By measuring these frequency shifts, the researchers had been additionally ready to calculate the force critical to break the bond. A passe copper probe tip broke the iron-carbon bond with a preferrred force of 150 piconewtons. With one other carbon monoxide molecule linked to the tip, the bond became broken by a hideous force of 220 piconewtons. To delve into the premise for these variations, the group passe quantum simulation learn the approach to mannequin changes within the densities of electrons in the end of chemical reactions.
The work takes merit of AFM technology first advanced in 2009 to visualize single chemical bonds. The managed breaking of a chemical bond the employ of an AFM system has been more demanding than identical examine on bond formation.
“It is a immense downside to beef up our idea of how chemical reactions would possibly perhaps also be implemented by atom manipulation, that’s, with a tip of a scanning probe microscope,” acknowledged Leo Wicked, who leads the Atom and Molecule Manipulation examine group at IBM Study in Zurich, and became the lead creator of the 2009 ticket that first resolved the chemical structure of a molecule by AFM.
By breaking a selected bond with varied pointers that employ two varied mechanisms, the fresh ticket contributes to “making improvements to our idea and management of bond cleavage by atom manipulation. It adds to our toolbox for chemistry by atom manipulation and represents a step forward toward fabricating designed molecules of accelerating complexity,” added Wicked, who became now not fervent with the ticket.
The experiments are acutely wonderful to external vibrations and other confounding factors. The Imaging and Diagnosis Center’s specialized AFM instrument is housed in a excessive-vacuum ambiance, and the materials are cooled to a temperature of 4 Kelvin, factual a few degrees above absolute zero, the employ of liquid helium. These managed prerequisites yield honest measurements by ensuring that the molecules‘ vitality states and interactions are affected greatest by the experimental manipulations.
“You want a essentially excellent, neat system because this response would possibly perhaps presumably be very complicated—with so many atoms fervent, you wouldn’t know which bond you break at this kind of little scale,” acknowledged Yao. “The beget of this technique simplified your complete route of and clarified the unknown” in breaking a chemical bond, he acknowledged.
The ticket’s lead authors had been Pengcheng Chen, an companion examine pupil at PRISM, and Dingxin Fan, a Ph.D. pupil at the College of Texas-Austin. As successfully as to Yao, other corresponding authors had been Yunlong Zhang of ExxonMobil Study and Engineering Firm in Annandale, New Jersey, and James R. Chelikowsky, a professor at UT Austin. Moreover Arnold, other Princeton coauthors had been Annabella Selloni, the David B. Jones Professor of Chemistry, and Emily Carter, the Gerhard R. Andlinger ’52 Professor in Energy and the Environment. Assorted coauthors from ExxonMobil had been David Dankworth and Steven Rucker.
Extra facts:
Breaking a dative bond with mechanical forces, Nature Communications (2021). DOI: 10.1038/s41467-021-25932-6 , www.nature.com/articles/s41467-021-25932-6
Quotation:
Personnel measures the breakup of a single chemical bond (2021, October 4)
retrieved 5 October 2021
from https://phys.org/facts/2021-10-group-breakup-chemical-bond.html
This doc is subject to copyright. Other than any gorgeous dealing for the rationale of non-public ticket or examine, no
share would possibly perhaps presumably presumably be reproduced without the written permission. The boom is equipped for facts capabilities greatest.